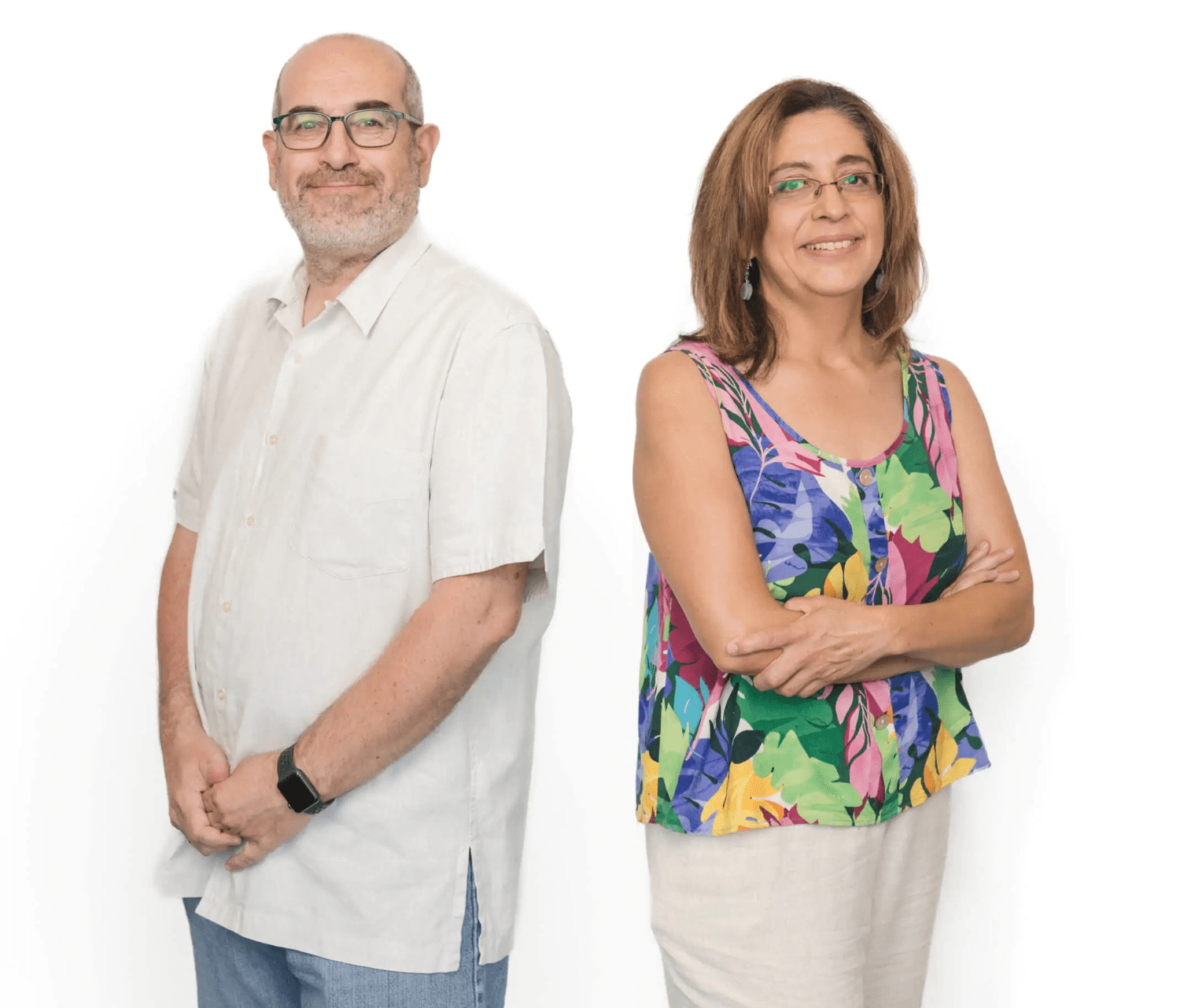
The effect of Fe(3)O(4) biosynthesized through the green synthesis of Silybum marianum and HA in the targeted delivery of 5-Fluorouracil to HCT116 cell line.
Autores: Mansuryar A, Bourang S, Noruzpour M, Ebrahimi HA, Amani A, Granados-Principal S, Calahorra J
07/2025 - Daru : journal of Faculty of Pharmacy, Tehran University of Medical Sciences